Current research activity in the Uludag Lab is comprised of two avenues of integrated explorations, one focused on polymer engineering for nucleic acid binding and condensation, and the other on cancer therapy by siRNA. There is a significant overlap (and continuity) in working paradigms of these research themes, and the activity in each area strongly complements the other research avenue. Our core expertise remains the synthesis of functional materials for modulation of cellular responses for a beneficial therapeutic outcome.
Polymer Engineering for Nucleic Acid Therapeutics
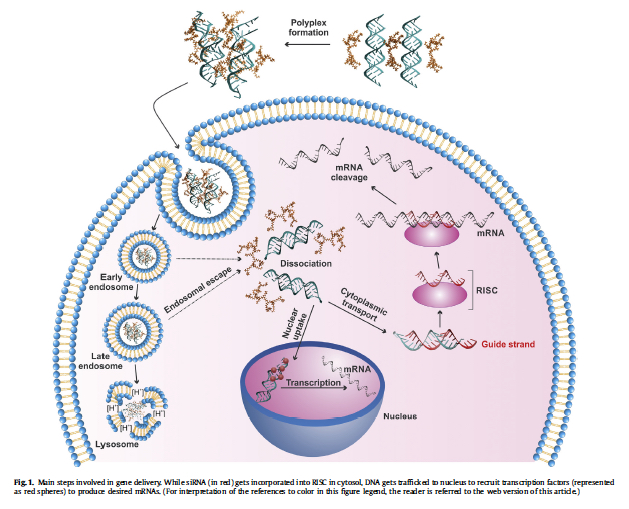
Extensive efforts by academic and industrial groups are generating new and exciting information about the role of various nucleic acids in human diseases. Expression systems for proteins (e.g., plasmid DNA and mRNA) and silencing molecules for protein expression (e.g., siRNA), for example, have been produced in large quantities and are now clinically employed for treatment of various human diseases.
However, current vehicles to deliver the nucleic acid especially in a clinical setting are limited in scope. A better approach to delivery of nucleic acids will significantly expand the scope of nucleic acid therapeutics. To develop next-generation delivery vehicles, we are engineering novel polymeric species that can interact with nucleic acids in a controlled way and modulate the exposure of cells to therapeutic nucleic acids.
The critical challenge is to generate simple enough chemical vehicles that do not disrupt the cellular physiology in a significant way, while delivering the nucleic acids to specific tissues in live organisms. We are creating new biomaterials to this end using new functional groups and taking advantage of emerging chemical synthesis schemes. While experimental approaches are extensively utilized to better understand the behavior of new vehicles, we are relying on molecular dynamics simulations to probe the interactions of new vehicles with cellular systems at atomistic scale. The details of our research could be found on the publications page of the web site.
We are always on the look out for diligent researchers to join our team; proven experience in polymer science and engineering and/or molecular dynamics simulations are what we are looking in aspiring scientists.
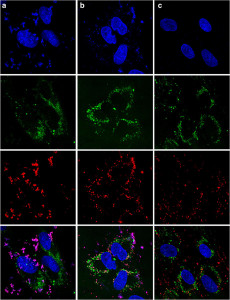
Cancer Therapy Using siRNA
Cancer arises from uncontrolled cell proliferation due to aberrant expression of select set of proteins, resulting in a loss of control over the normal cellular physiology. Most conventional cancer drugs do not target these changes in the cells, but rather aim to curb uncontrolled cell proliferation non-specifically. A permanent solution to uncontrolled cell proliferation is to target those proteins with aberrant expression and silence the mediators responsible for this effect. The silencer RNA molecules (also known as siRNA) are ideal agents for this purpose. They can be designed to target any protein at will and they can form an effective platform to target multiple aberrant proteins simultaneously. With the novel delivery systems generated in Uludag lab, we are tackling aberrant gene expression in human cancers. We are targeting select transcription factors and oncogenes in human leukemias to eradicate the transformed cells.
Our goal is to design ‘nano’-engineered vesicles, based on architecturally-controlled polymers, to facilitate cellular uptake of siRNA and protect it from degradation. We are particularly interested in amphiphilic polymers composed of cationic groups and lipophilic groups, since these features provide an optimal balance between packaging of siRNA into nano-vesicles and cellular penetration. In addition, we plan to add bioactive moieties onto the vesicles that can impart cell-selectivity to our delivery systems, so that cancerous cells are targeted in the body, rather than the normal, healthy cells. More information about our siRNA research can be found in our publications available in the Publications page.
We recruit budding scientists with solid understanding of cancer physiology and nucleic acid pharmacology to contribute to our efforts. Experience with ‘liquid’ tumors and/or animal models are desirable to join our cutting edge research projects.